Snakeheads (genus Channa) are one of the best known and most sucessful predatory freshwater fishes in Southeast Asia, but surprisingly, little is known of their natural history and biology. They are renowned food fishes, and are popular in most markets. Many of the smaller and more exotic species are also prized as aquarium fishes, commanding high prices. The genus Channa thus, is valuable both as food and as aquarium fishes. Some snakeheads are also important as sport-fishes and as pests in fish farms. They are also called snake-headed fishes, serpent-headed fishes or murrels in other parts of Asia. In Malaysia, they are known as Ikan Toman, Ikan Haruan, Ikan Aruan, Ikan Bujok and Ikan Bakak, depending on the species (see Tweedie, 1952a, b; Lim & Ng, 1990).
Channa are air-breathing freshwater fishes with some 30 species known from Africa and Asia ( Myers & Shapovalov, 1931 ; Wheeler, 1985; Roberts, 1989). There are however, a large number of subjective synonyms which will have to be checked. The Asiatic species range from Afghanistan through to India, Sri Lanka, Burma, Indo-China, China, Japan, Taiwan, Southeast Asia, including the Lesser Sunda Islands, Philippines and Sulawesi. The family is absent from Australasia. Southeast Asia appears to be the centre of diversity, with some 10 known species. Nine of these occur in Singapore, Malaysia and Indonesia (part). Five species, viz., the Giant Snakehead, Channa micropeltes ; Common Snakehead, C. striata ; Forest Snakehead, C. lucius ; Black Snakehead, C. melasoma and Dwarf Snakehead, C. gachua , occur in Singapore.
Historically, Peters (1868) described a new subspecies, Ophiocephalus guachua var. malaccensis (spelling of guachua incorrect) from Songei Kranjei, nördl. von Singapore (p. 262). He however, appeared to have had available only a single specimen. His subspecies is now generally regarded as a junior synonym of C. gachua . Interestingly, if the Southeast Asian specimens referred to C. gachua at present should prove to be distinct from the Indian specimens (see next section), this name might well become available.
The present article is designed to collate what is known about the biology of Southeast Asian channids, particularly the Singapore species, and to present the potential in deriving a better understanding of their biology. ToC
Much attention has been paid to the taxonomy of snakeheads in the past 60 years. Most of the older literature refer to the genus as Ophicephalus Bloch, 1793 (e.g. Herre & Myers, 1937; Smith, 1945; Boeseman, 1949 etc.). More complications arise as this name is often incorrectly spelled Ophiocephalus (e.g. Hamilton-Buchanan, 1822 ; Karoli, 1822; Peters, 1868 ; Day, 1878-1888 , 1889 ; Weber & de Beaufort, 1922 etc.). The name Channa Scopoli, 1777 finds its place only in more recent works. Even this name has had problems as it was first used by Gronovius (1763) , but that name is a nomen nudum. It was subsequently used correctly by Scopoli (1777) , although a type species was only designated 24 years later by Bloch and Schneider (1801) .
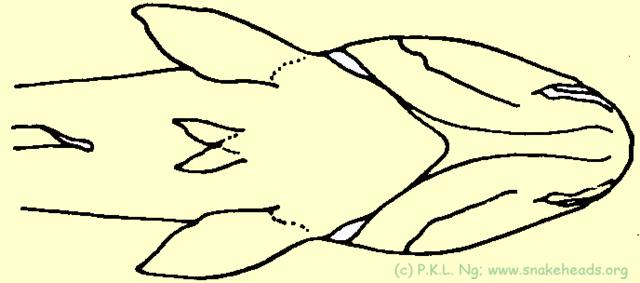
 | Fig. 5a: Schematic drawing of the genus Ophicephalus: the two ventral fins between the pelvic fins was the sign of the genus Ophicephalus, introduced by M.E. Bloch, 1793. |
Myers & Shapovalov, 1931 suggested that the presence or absence of pelvic fins, being variable, was not important as a character for differentiation, even between species. They commented that the two closely related species, C. orientalis and C. gachua Hamilton- Buchanan, 1822 (which has pelvic fins) were actually synonyms, and the absence of the fin was by loss, and not by design ( Fig. 5A , Fig. 5B ). As orientalis was the older name, it had precedence over C. gachua . Hence Channa and Ophicephalus cannot be distinct. This view was adopted by many ichthyologists of that time, including Smith (1945) who however made a plea to retain the better known name of Ophicephalus over its legal older name as dictated by the rules of nomenclature. The rules of nomenclature however, had to be followed nevertheless.
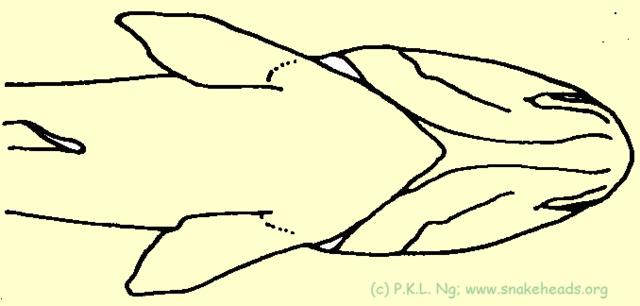 | Fig. 5b: Schematic drawing of the genus Channa: the missing two ventral fins between the pelvic fins was the sign of the genus Channa, introduced by A.J. Scopoli, 1777, Gronovius 1763 respectively. |
A prominent dissenter to the synonym of C. gachua and C. orientalis was Sri Lanka's Deraniyagala, who in three papers ( 1929 , 1932, 1963), provided strong evidence that the absence or presence of pelvic fins was genetic - not accidental. The two are otherwise extremely close morphologically. Deraniyagala based his conclusions on extensive field work and examination of numerous specimens, and the evidence was far stronger than Myers & Shapovalov, 1931 . Yet, Deraniyagala's findings were generally ignored until the 1980s. Myers & Shapovalov's observations influenced a whole generation of ichthyologists, and resulted in the synonymisation of numerous species and forms under the catch-all name of Channa (or Ophicephalus) orientalis (see DeWitt, 1960; Alfred, 1961, 1966, 1971; Roberts, 1989; Kottelat, 1989).
Deraniyagala ( 1929 , 1932) observed that all the young of gachua and C. orientalis follow the characteristics of their parents. He commented that despite Day's (1878-1888) observation that «it was not uncommon in lndia to find specimens of Ophiocephalus gachua having a ventral fin deficient but I have never observed both wanting» ( Day. 1878-1888: 368 ) (which was used as supporting evidence in Myers and Shapovalov's 1931 paper ), this was certainly not true in Sri Lanka. Myers and Shapovalov , while citing Deraniyagala's ( 1929 ) observations that the two species were very similar, failed to note that he had numerous specimens on hand and no anomalous ones were found.
Myers and Shapovalov (1931) also citedHora's (1921) paper in their comments that the loss of pelvic fins is a well-known phenomenom (p. 34). They however, neglected to report Hora's conclusions correctly.Hora (1921) writes: The only character, therefore, that distinguishes Channa from Ophiocephalus is the absence of the ventral fins. The occasional absence of the ventrals has been regarded in other genera as an abnormality or a case of genetic variation; but in Channa this character seems to have become permanent, for large series of specimens with the ventrals absent have been collected from the same locality (p. 31) I conclude, therefore, that the cases of Apua [a cobitid] and Channa are not to be considered parallel. Channa has been found by numerous collectors at many different places over a wide area and the ventrals are invariably absent. Apua, on the other hand, has only once been collected and only two individuals were then found. (p. 32). In a later paper, Hora and Mukerji (1934) synonymised C. harcourtbutleri Annandale, 1918 with C. gachua and reported an aberrant C. gachua from Burma. The aberrant specimen is interesting because although it lacks both pelvic fins, rudiments of the fin and the basipterygia are still present. Their dissections of C. orientalis from Sri Lanka showed these structures to be absent. They suggested that the Burmese specimen which lacked the pelvic fins had lost the fins through injury or disease (p. 137) whereas the absence of the pelvic fins in C. orientalis was natural. Jayaram (1981: 306) incorrectly noted that it was Hora and Mukerji (1934) who synonymised the Indian C. orientalis and C. gachua - they had merely commented on their close similarity.
Ng and Lim (1989) noted that none of the specimens of C. gachua they have examined from Malaysia and Singapore are abberant with regards to the pelvic fins. This is also true for specimens of C. gachua the authors have examined from Indo-China, Kalimantan, Sumatra and Java (unpublished data). A German aquarist, Günther Ettrich (1982, 1986 , 1989a, b), who first managed to breed C. orientalis and C. gachua from Sri Lanka in the aquaria, noted that all the offsprings of C. orientalis lacked the pelvic fins of the parents, supporting Deraniyagala's belief that the differences observed were genetic. Myers and Shapovalov's (1931) comment that ... it seems reasonable to suspect that Channa orientalis may be nothing but a series of anomalous specimens of C. orientalis, a species which in certain streams of Ceylon, more than elsewhere, shows a tendency to lose its pelvic fins ( p. 34 ) should thus be taken as it stands - a suspicion no more, and as the bulk of evidence seems to indicate, an incorrect one at that. Kottelat (1989), while using Myers and Shapovalov's (1931) concept of orientalis , admitted that it was probably incorrect, and the lumping of gachua and a host of other synonyms with orientalis had been unjustified.
Kottelat and the present authors now share the belief that C. gachua is clearly distinct from C. orientalis although the similarity between both species is remarkable (see Ng & Lim, 1989; Lim & Ng, 1990; Lim et al., 1990a, b). Zakaria-Ismail (1984) also uses C. gachua rather than C. orientalis . To make matters even more complicated, what we call C. gachua is might not even be that species. The original Channa gachua was described from India, and there are indications that the Southeast Asian and Indian species are different. All the synonyms will need to be critically checked and the original material examined before these problems can be resolved. Considering the geographical distribution of C. gachua/C. orientalis and its complex of species ranges from Afghanistan to Bali, including Burma, India, Taiwan and Sri Lanka - this is no mean feat.
A final note about the absence or pelvic fins seems pertinent. Myers and Shapovalov (1931) did not consider one species which is also crucial (or perhaps fatal?) to their case Channa asiatica (Linnaeus, 1758) . Like C. orientalis , C. asiatica consistently lacks pelvic fins (see Wheeler, 1985; Hay & Hodgkiss, 1981). The synonyms associated with C. asiatica will also have to be re-examined.
On a broader perspective, it is still uncertain if the three African species belong to the same genus as the Asiatic taxa. Roberts (1989) noted that the genus Paraphiocephalus Senna, 1924 is available for the African species, but as yet, remains poorly defined. Most ichthyologists prefer to classify the African species in the genus Channa for the time being. ToC
The channids have always been closely associated with the labyrinth fishes. They have been classified as a suborder (Channoidea) in the Order Labyrinthici or Ananbantoidei (e.g. Weber & de Beaufort, 1922 ; Deraniyagala, 1929 ). Some authors (e.g. Günther, 1861 ; Berg, 1940; Liem, 1963) however, have expressed opinions that the Channidae are not anabantoids. Roberts (1989) on the other hand, argues strongly in favour of the anabantoid relationship of channids. He writes - «Having now reviewed the biology and examined radiographs and osteological preparations of many anabantoids and Channidae, I feel the best available hypothesis is that snakeheads are anabantoids. The functional significance of the posterior extension of the swim bladder present in all anabantoids may be to provide an adjustable counterpoise to the airbubble in the suprapharyngeal respiratory organ (W. C. Freihofer, pers. comm.). I predict that evidence supporting the broad phyletic relationships among anabantoids outlined here will accumulate in proportion to comparative observations on their morphology, development, physiology and behavior. The recent observation that Channa gachua [nec Channa striata] is an oral brooder (Ettrich, 1982) is relevant. I suggest that this is further evidence of the anabantoid relationships of Channidae, and that detailed observations of its oral-brooding and comparisons with oral-brooding in other fishes will bear this out. The distinctive spawning embrace characteristic of numerous anabantoids apparently also occurs in Channa.» (Roberts, 1989: 20).
All our preliminary observations and studies of Southeast Asian channids support Robert's observations, and we are also inclined to accept channids as closely related to, if not part of, the anabantoids. A precise classification however, is still wanting. ToC
The main taxonomic characters used in channid taxonorny include scale counts, the presence or absence of large canines on the vomer and palatine, pelvic fins (see earlier), and a black ocellus on the tail and proportions of the pectoral fins to the head. The use of the caudal ocellus may not always be reliable as it is obscure but still just visible (on a dark background) in C. melanoptera . The canines are reliable only in adults, the condition in young specimens being less distinct. Colour patterns are useful, but only for live specimens. The distinctive colour pattern of C. melasoma is assumed only when the animal is well conditioned. Freshly caught animals are very pale and uniformly coloured. Freshly dead specimens on the other hand, are uniformly very dark (which might have lead to the species being named melasoma ). The red margins of C. gachua become white on preservation or in some aquarium specimens. Dark colour patterns are sometimes useful. The very dark green to black pectoral fin base of C. gachua is always present, as is the black spot on the opercula of C. lucius , C. bankanensis and C. pleurophthalma . The concentric black rings on the pectoral fins of C. gachua and C. lucius are almost always distinct, being more obvious in C. gachua . The scattered small pale spots on the pectoral fins of C. lucius not always apparent, and disappears rapidly after preservation.
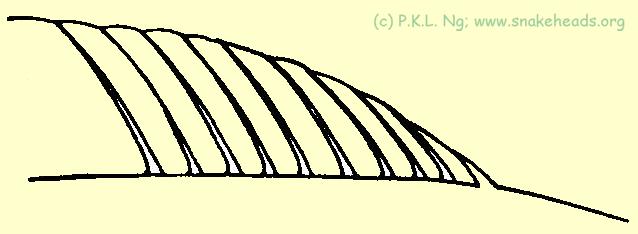 | Fig. 5g: Schematic proportional drawing of the dorsal fins of C. marulioides and C. melanoptera: the rays get progressively longer as moved to the caudal. |
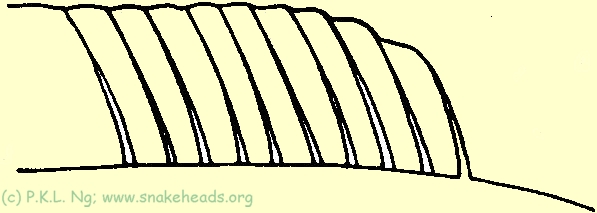 | Fig. 5f: Schematic proportional drawing of the dorsal fins of C. striata: the first ray is only slightly shorter the the 2nd and the subsequent rays are all subequal in length. |
Deraniyagala (1929) used the form of the head shields and structure of the cephalic sense pits to effectively separate the Sri Lankan species. The cephalic shields are especially useful in separating out Channa gachua (see Deraniyagala, 1929 ; Munro, 1955), and their figures agree very well with Malaysian and Singapore specimens of this species. We have also noted that the cephalic sense pits increases in complexity as species like C. striata grow, being simple pits in young fishes but becoming more sieve-like in large specimens. In C. melasoma and C. gachua however, the pits remain simple. This is especially obvious in C. gachua . The function, variation and value of these characters is being studied in detail for the Southeast Asian taxa.
We have also found the short nasal tentacle or tube extending from the nostrils to be useful in separating some species. This tube is the most well developed (relative to its body size) in C. gachua , and is distinct in adults of C. lucius , C. striata and C. melasoma . In C. micropeltes , the tube are evident only in the younger specimens. In adults, it becomes very reduced and indistinct. This character has been featured in some figures but hardly ever described. The tubes can be damaged through burrowing or terrestrial locomotion, and may be folded against the side (especially in preserved specimens), and are thus not always obvious. Its taxonomic value for the other species will have to be assessed.
Barring some of the above-mentioned taxonomic problems, nine species of Channa are known at present from Peninsular Malaysia, Singapore, Sumatra, Borneo and Java.
In the last revision of the freshwater fishes of Peninsular Malaysia, Mohsin and Ambak (1983) recognised nine species ( C. striata , C. micropeltes , C. lucius , C. bistriata , C. punctatus , C. orientalis , C. melanosoma , C. marulioides and C. gachua ) as present. This work however, does not take into account most of the modern developments and represents nothing more than an uncritical compilation of older literature (see also Zakaria-Ismail, 1983; and last section of present paper). Of their nine species, only six are valid ( see Conclusion ). Roberts (1989) recorded all nine species from the Kapuas drainage in western Kalimantan, Borneo, and noted that the area had the highest concentration of channids in Southeast Asia. The authors have examined specimens of all nine species, and the differences in body colour and morphology are quite obvious (Fig. X). Of these nine species, five occur in Singapore. Peninsular Malaysia has seven - C. marulioides and C. melanoptera occuring only in the northern half of Peninsular Malaysia, with the fauna of Johor and the southern states sharing the same fauna as Singapore. Channa pleurophthalma and C. bankanensis are known only from Sumatra and Borneo. Weber and de Beaufort (1922) and Roberts (1989) record C. bankanensis from parts of Borneo and the island of Banka (=Bangka) off southeast Sumatra. We have examined specimens from central Borneo and the Sumatra mainland. Boeseman (1949) also recorded a new fossil species, Ophicephalus palaeostriatus (ostensibly allied to C. striata ) from Java.
Some general taxonomic notes seem prudent. Of these species, C. bankanensis
 | Fig. 5 c: In comparison to C. bankanensis, C. lucia has a longer snout and its head is proportionally also longer. |
 | Fig. 5 d: In comparison to C. lucia, C. bankanensis has a more blunder snout and its head is proportionally shorter. |
 | Fig. 3 I: dorsal view of C. pleurophthalma. |
 | Fig. 3 J: ventral view of C. pleurophthalma. |
Channa melasoma and C. striata are superficially very similar, especially smaller specimens, but their head structures and especially the live colour patterns (of small juveniles and adults, are strikingly different (see Ng & Lim, 1990). The intermediate sized specimens are the ones which pose the most serious identification problems when dead.
Channa melanoptera and C. marulioides are both similar to C. melasoma , but are generally longer fish. Their first dorsal fin ray is also always distinctly shorter (about two-thirds length) than the second. From the specimens we have examined, C. melanoptera seems to be a generally larger and more brightly coloured species. While many authors separate these two species mainly by its smaller size, plainer colouration and the presence of an ocellus on the upper part of the caudal fin (absent in C. melanoptera ) (see Weber & de Beaufort, 1922 ; Tweedie, 1949), this character is less than ideal. We have examined very large specimens of C. melanoptera and found a faint but obvious black ocellus on the tail. The generally dark colouration of the species tends to obscure the ocellus, but it is usually there nevertheless. Smaller specimens of C. melanoptera generally have more distinct ocelli. The body scales and patterns appear to differ significantly. Scattered patches of dark-coloured or black scales along the midaxial line of C. melanoptera have a white outer border, which contrasts strongly with the black and orange scale pattern of the body. Such white-bordered scales are not known to be present on C. marulioides , the body colouration being more uniform (cf. Weber & de Beaufort, 1922 ). The black patches of body scales however, may well be due to variation or are sexual features. Certainly, in one Malaysian specimen of C. marulioides , several dull grey patches of scales can be discerned. Channa marulioides is poorly known and is represented by few specimens, none of which are very large. Weber and de Beaufort (1922) records the size range as up to 270 mm, and we have examined specimens from Peninsular Malaysia smaller as well as slightly larger than this. The Malaysian specimens of C. melanoptera we have examined are all (with one exception) longer than 270 mm. It may well be that what has been called C. marulioides is merely a colour-morph or juvenile of C. melanoptera . If this is so, then C. marulioides becomes the valid name. Some colour notes of an aquarium specimen of C. melanoptera seem pertinent. The dorsum is dark brownish-green, the lateral areas below the lateral line being predominantly yellow; the ventral surfaces being white. The brownish-green areas jut into the yellow areas in altemate patches. Many of the dark scales along the distal part of the body have a white margin at the edge of their scales. The black patches of scales mentioned by Weber and de Beaufort (1922) probably include these. The fins are bluishgreen, with small white flecks on the anal, caudal and dorsal fins. The colouration of the head is similar to the body, except that the upper part of the operculum has a large lightcoloured spot against a brownish-green background on each side. The cephalic shields of C. melanoptera are also very pronounced, the scales separated by deep grooves.
We have also examined specimens of a species from northern Sumatra which do not fit the descriptions of any known species. It is allied to C. melanoptera in body form, but differs in several important aspects, as well as having a very characteristic colour pattern It appears to be an undescribed species.
Much has been written about the dramatic changes in body colour and patterns between young fishes and adults. That of C. micropeltes has been documented and figured by Day (1878-1888) and Tweedie (1949) (Fig. 1A , 1B , 1C ), while Alfred (1964) showed that the species known as C. bistriata is merely a juvenile stage of C. lucius (Fig. 1G , 1H , 1I ). The changes in C. striata are already well known (see Smith, 1945), and Ettrich's (1982, 1986 , 1989a, b) studies provide data for C. gachua and C. orientalis . Recently, the young of C. melasoma were described (Ng & Lim, 1990) (Fig. 1D , 1E , 1F ), validating beliefs that C. rhodotaenia is probably a junior synonym (see Roberts, 1989).
Some brief nomenclatural notes about the following species are necessary:
No known synonyms. ToC
Synonyms: This species needs to be revised (see earlier), and the status of many synonyms are uncertain. Weber and de Beaufort (1922) listed the following synonyms: Ophicephalus aurantiacus Hamilton-Buchanan, 1822 ; Ophicephalus marginatus Cuvier, in Cuvier & Valenciennes, 1831 ; Ophicephalus limbatus Cuvier, in Cuvier & Valenciennes, 1831 ; Ophicephalus coramota Cuvier, in Cuvier & Valenciennes, 1831 ; Ophicephalus fuscus Cuvier, in Cuvier & Valenciennes, 1831 ; Philypnoides surakartensis Bleeker, 1849 ; Ophiocephalus kelaarti Günther, 1861 ; Ophiocephalus guachua var. malaccensis Peters, 1868 ; Ophiocephalus harcourt-butleri Annandale, 1918 . 1 The species name is sometimes incorrectly spelled as guachua (e.g. Peters, 1868; Sterba, 1973). ToC
Synonyms: Ophiocephalus polylepis [sic] Bleeker, 1852 ; Ophiocephalus bistriatus Weber & de Beaufort, 1922. Károli's (1882) species, Ophiocephalus bivittatus is synonymous with bivittatus C. lucius , but as the name is already preoccupied by Ophiocephalus bivittatus Bleeker, 1845 , Weber and de Beaufort (1922) changed it to bistriata . Both Weber and de Beaufort (1922) and Tweedie (1949) had commented that C. bistriata might be merely the young of C. lucius , but it was Alfred (1964) who showed this to be so. ToC
 | Fig. 1 g: coloring of C. lucius having a size of 4 cm. |
 | Fig. 1 h: coloring of C. lucius having a size of 6 cm. |
 | Fig. 1 i: coloring of C. lucius having a size of 21 cm. |
No known synonyms. ToC
No synonyms, but has often been confused with Channa marulius Hamilton Buchanan, 1822 . The presence of this species in Java (Bean & Weed, 1912: 607) was challenged by Weber & de Beaufort (1922: 316) , and there have been no confirmation that this species is in fact there. ToC
Synonyms: Ophicephalus rhodotaenia Bleeker, 1851; Ophicephalus mystax Bleeker, 1853; Ophiocephalus baramensis Steindachner, 1901. 2 Frequent incorrect spelling: Channa melanosoma. ToC
 | Fig. 1 d: coloring of C. melasoma having a size of 14 cm. |
 | Fig. 1 e: coloring of C. melasoma having a size of 0.7 cm, top view. |
 | Fig. 1 f: coloring of C. melasoma having a size of 0.7 cm. |
Synonyms: Ophicephalus serpentinus Cuvier, in Cuvier & Valenciennes, 1831 ; Ophiocephalus bivittatus Bleeker, 1845 ; Ophiocephalus stevensii Bleeker, 1853 ; Ophiocephalus diplogramme Day, 1865 ; Ophiocephalus studeri Volz, 1903. ToC
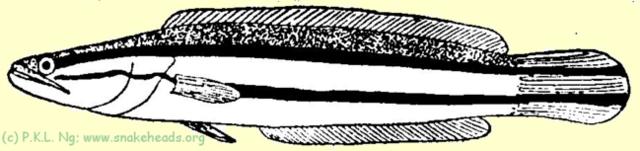 | Fig. 1 A: Schematic drawing of C. micropeltes showing coloring at the size of 15 cm. |
 | Fig. 1 B: Schematic drawing of C. micropeltes showing coloring at the size of 28 cm. |
 | Fig. 1 C: Schematic drawing of C. micropeltes showing coloring at the size of 60 cm. |
Synonyms: Ophicephalus urophthalmus Bleeker, 1852 , possibly Ophicephalus spiritalis Fowler, 1904 . Tweedie (1940: 78) reported this species from Malaysia, but later (1949) corrected his identification and referred the specimen to C.lucius . Channa pleurophthalma is thus absent from Peninsular Malaysia. ToC
 | Fig. 4 l: Side view of C. pleurophthalma (in spirit). |
 | Fig. 4 f: Side view of C. pleurophthalma. |
** Synonyms: Ophiocephalus wrahl Lacépède, 1802, Ophiocephalus chena Hamilton- Buchanan, 1822 ; Ophicephalus planiceps Cuvier, in Cuvier & Valenciennes, 1831 ; Ophicephalus sowarah Bleeker, 1845; Ophiocephalus cyanospilos Bleeker, 1853 3 ; Ophiocephalus vagus Peters, 1868 . ToC .
Two other species previously recorded from Peninsular Malaysia or Singapore, Channa orientalis s. str. and C. punctata Bloch, 1793 are regarded as erroneous records ( Weber & de Beaufort, 1922 ; Fowler, 1938; Alfred, 1966; Ng & Lim, 1990).
The best available key to the Indo-Australian channids is still that by Weber & de Beaufort (1922: 314-315) . The key by Smith (1945: 466-467) includes some Thai species not known from peninsular and insular Southeast Asia. Tweedie's (1949: 100-101) key to the Malaysian species draws heavily from Weber and de Beaufort's. We have constructed the following key to supplement that of Weber and de Beaufort (1922) . While scale counts, proportions of the fins and presence or absence of caniform teeth on the upper jaw remain useful, we have, for practical purposes, reduced their emphasis here, and appended several other more 'accessible' characters which we have found useful. A separate key has been designed for the juveniles which are known. For these, generally only the younger stages are considered, and the gradual changes in the colour patterns of the intermediate-sized individuals have been omitted.
Key to the identification of adult channa
Key to the identification of juvenile channa
Channa micropeltes , C. striata and C. lucius are highly prized food fishes (in this order of preference), and are sold in large numbers in most wet markets in this region (including Singapore). They command very high prices of between S$10 and S$20 per kilogramme when fresh. Locally, they are particularly sought after by snakehead meat vermecelli by the Chinese, as well as cooked in a variety of dishes by the other ethnic groups.
Of freshwater food fishes, they are amongst the easiest to transport due to their airbreathing abilities and hardiness. Mortalities on transit are generally low. In local markets, they are kept in large bins or tubs in large numbers in little water without aeration. They are netted or hand-removed when needed. Surprisingly, even large C. micropeltes do not resist very fiercely when man-handled for slaughter. They are killed by blows to the head with a piece of wood which is sometimes spiked at one side. The fishes are scaled and the meat removed from the skeleton with the head intact. The head and the skeleton are used in soups. They are also popular in restaurants, the fish kept live in tanks until they are needed for cooking.
With firm hard meat and supposed medicinal value (especially for post-surgical patients), supply rarely meets demand. There are many published reports about the delicacy of C. striata and how much it is valued as a food fish ( Willey, 1909 ; Deraniyagala, 1929 ; Smith, 1945; Soong, 1949; Lim & Ng, 1990 etc.). In other parts of Asia (e.g. Burma and Sri Lanka), species like Channa gachua and C. melasoma are also eaten. Although C. gachua is a small species, it is nevertheless consumed, or sometimes used as bait-fish to catch larger channids like C. striata (see Deraniyagala, 1929 ). Most of the local supplies of C. striata , C. micropeltes and C. lucius are imported from Peninsular Malaysia and Indonesia, although small scale farming of purchased juveniles does occur in some rural areas of Singapore. As far as is known, controlled breeding of any species not practised. ToC
Being a valuable food fish and pest may seem contradictory to the many reports which have praised them as valuable animals. The culture of aquarium and food fishes like Koi, Goldfish, Guppies, Mollies, Red Tilapia etc. however, can suffer greatly if snakeheads, C. micropeltes and/or C. striata , are in the ponds, especially the former (see Lim & Ng, 1990). In Singapore, the introduction of valuable carp or other fishes into large ornamental ponds often necessitates the removal of the snakeheads by the draining of these ponds. The same procedure is necessary in cases where snakeheads have inadvertently entered cultured stocks of aquarium fishes. ToC
The value of snakeheads as food is also complimented by the fighting spirit of the fish when hooked, especially of the giant C. micropeltes . There are many reports of the strength and sport-worthiness of this fish (e.g. see Smith, 1945). In many angling areas, C. micropeltes is intentionally introduced for sport. Baits used range from frogs, pieces of meat, poultry viscera to small live ducks and fish. ToC
Several of the smaller and more colourful local Channa species are sought after for aquarium trade. Channa gachua easily fetches prices of between S$30 to S$60 per adult fish. They are popular due to their small size and brightly coloured fins. Related colorful species in Borneo, Sumatra and Malaysia (mainly C. melanoptera and C. pleurophthalma ) command prices of up to S$100 per fish. Trade names include the Green snakehead ( C. gachua ) and Emperor Snakehead ( C. melanoptera ). The Emperor snakehead is frequently identified locally as C. marulioides . Occasionally, even smaller C. lucius are offered for sale in local aquaria, as well as Chinese species like C. asiatica .
As far as is known, all aquarium stocks are from the wild, and continued collections (if it has not already) threaten their existence. This problem is especially serious considering that the sought after forms are wholly forest species and deforestation is realy a serious problem in their native habitats. Some of the species like C. gachua have relatively small brood size (unpublished data) and this further aggravates the threats. Occasional and random breeding successes have been reported for C. gachua by a German aquarist (see Ettrich, 1989a, b), but large scaled controlled breeding is still not known and the breeding behaviour and requirements of many other species is poorly, if understood. ToC
In the past few years, we have made numerous observations of habits and biology of some local species. As many of these observations have not been previously reported in nature it might perhaps be useful to document them to aid future studies on these species
While channids are known to be air-breathing fishes, probably closely related to the anabantoids, it has long been assumed that the airbreathing or suprabranchial cavity above the gills of channids is less developed compared to the other labyrinth fishes. Weber and de Beaufort (1922) makes the following diagnosis of the Channidae - In Ophiocephalidae the suprabranchial cavity is more like a diverticulum of the pharyngeal cavity, as it is in open communication with it. It is also without a labyrinthiform organ but covered by a mucous membrane [sic] fit for accessory respiration ( 312 ). This is certainly a generalisation. Deraniyagala (1929) (who regards the air-breathing cavity as an accessory pharyngeal air cavity and not the same as the suprabranchial cavity of labyrinths) amends Weber and de Beaufort's diagnoses when he writes ... transpiration occurs through the vascular mucous membrane which lines these cavities, the surface of which is increased by the presence of ridges and papillae ( p. 80 ). For the local species, large C. micropeltes have highly vascularised papillae all over the surface of the cavity, vaguely resembling the arborescent structure of the airbreathing clariid catfishes. It is also present, to a lesser extent in C. striata , C. melasoma and C. gachua . Smaller specimens of all these species however, lack the papilliform structures. Only in C. lucius is the cavity simple . This is not unexpected as many channids can be very active animals, especially when hunting, and such structures will greatly aid oxygen uptake.
How the suprabranchial cavity functions is uncertain. Each cavity is approximately divided into two parts by a muscular flap (see also Munro, 1955: 99, Fig. 11) arising from the edge, but how this flap works is not known. Air exchange in channids is via the mouth, not through the operculum (except inadvertently when hunting prey or disturbed). The roof of the mouth has a narrow groove which probably aids this process. The muscular flaps of the suprabranchial cavity are likely to be associated with the respiratory mechanism. ToC
The habitats of the five species is extremely interesting, and detailed studies in their ecological preferences will certainly have dividends. In open country areas, only two species can be found - C. striata and C. micropeltes . In shallow areas overgrown wit: grass and vegetation (averaging one metre of water or less), C. striata is the main species. We have encountered many specimens and their fry in canals, drains, ponds, small streams etc. They seem equally adept in stationary as well as slow flowing waters, but seem more common in the former habitat. This agrees well with reports that they art common in padi fields. Channa micropeltes on the other hand, is a pelagic fish, and prefers larger and more open bodies of water. Its native habitat is large lakes and river, but also adapts very well to large reservoirs and ponds with stationary water. Schools of young C. micropeltes however, have been found in slow flowing streams. Whereas C. striata tends to lurk among vegetation ambushing its prey, C. micropeltes seems to be wandering hunter, using occasional bursts of speed to capture pelagic fishes.
 | Fig. 2: C. melasoma in its habitat favors forested areas with slow flowing waters with submersed roots. |
Other than the air-breathing cavity, some channids also appear to have relatively well developed locomotory abilities on land. While walking fishes like Anabas and Clarias (see Smith, 1945; Ng et al., 1987 etc.) have been frequently reported upon, walking snakeheads are less well known. Deraniyagala (1929) , Smith (1945), Munro (1955), Mohsin and Ambak (1983) all note that the fish can move across land. Smith (1945) even records that C. striata can aestivate to a limited degree under mud when ponds dry out. There are however, no other reports, and we have not observed, that local C. striata , or any other snakehead does this. Mohsin and Ambak (1983) noted, that C. striata ... can survive in small holes and crevices of a pond which is drained prior to fish culture; all it needs is a little water inside the crevice (p. 158). The most adept at walking seems to be C. gachua . Specimens can move a considerable distance on all kinds of surfaces, squirming and skipping. Deraniyagala (1932) has commented they move in leaps. They are able to move in a straight line, alternately twisting their body to and fro, balanced, and possibly dragged along by their low-set pectoral fins. Channa melasoma and C. striata move across land in a similar fashion. The ventral scales before the pelvic fins in these species tend to be very smooth. It is tempting to speculate that the loss of the pelvic fins in species like C. orientalis might perhaps be related to their terrestrial abilities. The agility of these three species on land comes as no surprise, as all tend to inhabit shallow waters. Not unexpectedly, the species which lives in the most precarious habitat - very shallow streams - ( C. gachua ) is also the most agile. This habit probably explains their presence in seemingly inaccessible pools. Channa micropeltes and lucius on the other hand, are extremely clumsy on hand, and basically incapable of sufficient or directional terrestrial movement.
The form of the body and pectoral fins is a rough guide to the prowess of these animals on land. In transverse section, the bodies of C. micropeltes and C. lucius appear evenly rounded, whereas those of C. striata , C. melasoma and C. gachua are more dorso-ventrally flattened, the ventral surfaces being flatter. The approximate position of the pectoral fins is also indicative those in C. micropeltes and C. lucius are directed more horizontally and the bases of both fins are unable to touch the ground simultaneously. This is not the case for C. striata , C. melasoma and C. gachua , which have their pectoral angled in such a way as they can prop up the body and assist in crawling. These features are in agreement with observations of their habits C. micropeltes and C. lucius being mid-water to surface fish whereas C. striata , C. melasoma and C. gachua tend to be bottom dwellers. ToC
All channids are voracious predators. Prey is not exclusively fish, and small birds, amphibians etc. have also been reported to be eaten by the larger species (e.g. C. micropeltes ). The most ravenous is believed to be C. micropeltes , and Smith (1945) reports that they will sometimes kill more than they eat. In Singapore, we have once observed three sub-adult C. micropeltes harassing and mortally injuring a carp ( Cyprinus carpio ), almost as large as the snakeheads themselves by repeatedly trying to swallow it and spitting it out again as it was too much of a mouthful. The form of the teeth is interesting. While all the species possess numerous sharp teeth for gripping prey, some, like C. striata and C. micropeltes have enlarged caniform teeth. Even C. gachua has these caniform teeth. The caniform teeth of C. striata and C. micropeltes are also quite different when examined carefully. That of C. striata is cylindrical in cross-section, not like a dog's canine - ideal for gripping, killing and tearing. The caniform teeth of C. micropeltes more closely resembles a knife with two cutting edges in cross-section, the cutting surfaces being perpendicular to the animal's longitudinal axis. We have observed micropeltes catching fishes ( Oreochromis mossambicus ) obviously too large to be swallowed whole. The snakehead then shakes its head violently left and right, and the prey gets (literally) sheared in two! The cuts are relatively smooth and not very jagged, and certainly not due to tearing. The blade-like caniform teeth in C. micropeltes easily explains this. The teeth of the other species remain unstudied. ToC
Other than C. micropeltes , we have observed that the other Malaysian species are predominantly nocturnal hunters; especially C. gachua , C. lucius and C. melasoma . Channa micropeltes hunts mainly in the day, although fishermen do get them occasionally at night. Channa striata and C. lucius tend to move at dusk. Channa melasoma and C. gachua are almost wholly nocturnal. We have collected numbers of both these species mainly after dark. This probably explains why the latter two species were either believed extinct or not previously found in Singapore (see Ng & Lim, 1989, 1990).
The habits of the some species are worth noting. To catch C. striata , C. lucius and C. melasoma at night, the fishes can easily be dazzled by light, and chased into a net placed in their path. Once in the net, they keep trying to push their way through and are easily caught. We have also caught these species (and occasionally younger C. micropeltes ) by placing nets downstream and trashing the vegetation along the bank upstream. Channa melasoma does not always move from cover - it more often than not burrows its way deeper into the submerged vegetation and mud ( see Habitat ). Channa gachua reacts somewhat differently. When chased violently, the fish tends to move towards the source of the disturbance; and even when the fish enters the net, it quickly back-tracks . Most of our specimens were caught at night. After the fish have been sighted, they are gently coaxed (with our hands) into the net which is then quickly lifted. ToC
The species with the most cryptic body colour pattern is C. lucius , the form of the body, brown colouration with black blotches, allow the animal to blend very well into the aquatic debris of forest streams. It probably ambushes its prey with the aid of such patterns. Channa lucius is also only one of the three Southeast Asian Channa species which has a distinct large black spot on its operculum. Observations have shown that this spot is used in the same manner as the eye spot patterns on moths - to scare potential predators away. When disturbed, individuals with open their opercula wide and come face to face with the source of disturbance. From a distance, two large false eyes can be discerned (Fig. 5E ). Parents guarding their young also have been seen to perform this ritual. As far as is known, this has not been reported for any channid. The black opercular spots present in C. pleurophthalma and C. bankanensis (both absent in Singapore) may well serve the same function. As to the colouration of the adults, the longitudinal blotches on the side of the body which gives it its characteristic port-hole effect, may in some specimens, fuse to varying degrees, and appear as a jagged thick black stripe.
 | Fig. 5e: C. lucius frontal view. |
The colour of adult C. striata , and to a certain degree, C. melasoma , is quite dull, and helps hide the fish among its preferred habitat - submerged vegetation and debris. Adult C. micropeltes are strikingly coloured, but the role of these patterns is not known. The smallest species, C. gachua , is the most colourful. The dorsal, caudal and anal fins have an iridescent green base colour, the margins being bright red or orange. The pectoral fins are also distinctively striated semi-concentric rings, the base of the pectoral fins being dark green to black. The rich colours of this species possibly have a role in courtship, as males are always more intensely coloured. The colours are easily affected by the quality of the water (see Deraniyagala, 1929 ). Animals living in more acidic, leaf-litter laden and brownish waters tend to be darker coloured, the red in fins being more intense. Specimens in aquaria tend to lose their red colours, fading to orange and sometimes white unless sufficient leaf-litter is made available. ToC
Channids are well known for the fact that the colour patterns of their young are very different from the adults (see Day, 1878-1888 ; Weber and de Beaufort, 1922 ; Smith, 1945). Many of the synonyms reflected a lack of knowledge about such changes. For example, Channa bistriata was recognised as a valid species as .ate as the early 1960s before Alfred (1964) showed that it merely represented the young of C. lucius . Similarly, C. rhodotaenia is a synonym of C. melasoma ( Weber & de Beaufort, 1922 ; Ng & Lim, 1990). It is worthwhile to note that the changes in colour do not always occur at the same sizes, as noted by Alfred (1964). Tweedie (1949) noted that The species [C. bistriata] remains somewhat of a puzzle. No specimens exceeding 70 mm. standard length have been recorded and one is tempted to believe that they are the young of a normal sized member of the genus whose colour pattern changes, much as does that of C. micropeltes. Weber and de Beaufort (1922) suggested that they might be the young of C. lucius, but there are in this collection specimens of lucius of about this length and even smaller which show no sign of two pairs of black longitudinal stripes but have the two alternating series of lateral blotches characteristic of the adult . (p. 104). We have observed, collected and reared many specimens of C. lucius over the years, and in some cases, the young fishes assume the adult colouration at a smaller size than usual.
The reasons for these drastic changes in colour and pattern remain unknown, but is possibly associated with their schooling behaviour as young fishes. All the young have longitudinal stripes (which endures especially long in C. micropeltes and appears to be the shortest in C. gachua ) which may help break the outlines of the animals, and possibly confuse would-be predators.
The smaller individuals of species like C. striata , C. melasoma and C. gachua have well developed black ocelli at the base of their dorsal fins. These false eye-spots probably serve to confuse possible predators, similar to what is widely reported for the marine butterfly fishes. These ocelli usually disappear or become obscure as the fish grow. Deraniyagala (1929) reports many adult C. gachua and C. orientalis as having these ocelli, but in local specimens of C. gachua , these ocelli always disappear once the animals are mature. ToC
Sexing of channids is extremely difficult, and there are no known reliable external morphological characters. Colour is useful in C. gachua , the males been more brightly and intensely coloured. Size is also relatively useful as females tend to be larger and stouter. Even in well fed aquarium specimens (including C. gachua ), males tend to be more slender and smaller. An effective way to separate the sexes (and form potential breeding pairs) is to keep two individuals in a tank separated by a glass or mesh panel with an removable opaque screen. At regular intervals, the screen is removed and the response of the animals determined. If they show threat displays toward each other on every occasion, they are almost certainly of the same sex. Other than C. micropeltes , similar-sexed adults are highly aggressive towards each other. ToC
While schooling of young channids is usual, this is not the case for the adults. Only for Channa micropeltes have we observed small schools of adults - hunting not unlike aquatic wolf packs . Adults of the other species tend to be agnostic towards members of the same sex. Heterosexual pairs are frequently encountered.
As noted by Breder and Rosen (1966) and Roberts (1989), the young (particularly small fry) form tight schools when disturbed initially. We have observed this for C. micropeltes , C. striata , C. melasoma and C. lucius . This behaviour probably aids the parent in defending the young. If the intruder (e.g. man) persists however, and the parents are unable to do much, the young disperse in all directions. The young tend to aggregate in shallow slow-flowing water with thick vegetation. ToC
Interestingly, all channids reproduce in a similar fashion to many anabantoids.
 | Fig. 5h: C. gachua in courtship. |
 | Fig. 5i: C. gachua in spawning embrace. |
Perhaps the best known case of parental care in channids is that for C. micropeltes . Smith (1945) notes that this species is especially aggressive when guarding their young and are to be treated with extreme caution. Both parents care for their young, but the extent to which the parents are prepared to go to protect their young is not clear. We have collected fry of this species while half-submersed in water, but have not been attacked. There have however, been local reliable reports of serious injuries due to charging parent fishes. The size and bulk of C. micropeltes makes them especially dangerous. Both parents are also known to participate in fry-care in C. lucius . Other than visual threats, for example the displaying of opercular spots in C. lucius (see Role of colour); splashing of water at the intruder has also been observed. Parental care has also been observed for C. melasoma and C. gachua . In both cases, only one parent has been observed taking care of the fry. The parent C. melasoma may sometimes be a few metres from the fry. Channa gachua is a known mouth-brooder. Ettrich ( 1986 , 1989a, b) has recorded mouth-brooding (by males) for both C. orientalis and C. gachua from Sri Lanka, and the same is true of local C. gachua . One male, sheltered in a shallow burrow (not necessary of his making), was seen caring for 12 small fry. Another male disgorged 16 fry when captured. These observations seem to indicate that this species has a relatively small brood size. Special mention must be made of the dedication and altruistic nature of male C. gachua's care for their young. In one instance, the male, but not its young, had escaped the intentions of our net. He promptly turned back and went into the net in an attempt to nudge the young out. For C. striata , the parents are less often seen. It is uncertain if they are nearby but well concealed in the undergrowth or some distance away. Willey (1909) and Soong (1949) provide more information about parental care in C. striata . For all species of Channa, the parents will attack any fish which comes near the fry. ToC
In summary, it is prudent to note that the trade in snakeheads as both food and exotic aquarium fish is very large. There is an increasing demand for tropical Asian exotics especially carnivorous forms. A case in point is in the Golden Dragon Fish ( Scleropage formosus ) whose high value has resulted in natural stocks being critically depleted.
None of the Channa species in Southeast Asia are believed to be threatened with extinction. Alfred (1966, 1968) had indicated that C. gachua (as C. orientalis ) was extinct in Singapore but the species is still extant (Ng & Lim, 1989). Mohsin and Ambak's (1983 book on the Peninsular Malaysian fishes gives the highly incorrect impression that only
C. striata is still common, with C. micropeltes and C. lucius being endangered (p. 248), and C. bistriata (= lucius ), C. punctatus (= punctata ), C. orientalis (= orientalis ), C. melanosoma (= melasoma ), C. maruloides (= marulioides ) and C. gachua as rare or extinct (p. 249). Channa micropeltes is certainly not in any danger as it is widely cultured and abundant in all large bodies of water; and C. lucius remains common in many forested areas. Such uncritical generalisations are neither useful nor help conservation efforts.
In any event, it is the wholly forest species who potentially face the severest threats. In recent years, quantities of C. lucius have become available in local markets, the stocks of which are almost certainly from the wild. Whether such fisheries are sustainable remain to be seen. Channa gachua faces other problems. It not only appears to have more fastidious habitat requirements but also relatively smaller brood sizes. Its shallow water habitat also easily avails itself to collecting, especially at night. While this species has a wide range and appears to be quite successful, increasing deforestation and a growing aquarium trade will pose serious problems for it. ToC
The authors are most grateful to Dr. Maurice Kottelat for the many interesting and useful discussions we had on the subject, and his kind help. The staff of the zoology department, Bogor Museum were also most helpful in our studies, and kindly loaned us many of the specimens. Our work would not have proceeded so well had it not been for the generosity of the staff of the Zoological Reference Collection (National University of Singapore) in permitting us to examine the older specimens kept there at leisure. Thanks are also due to our colleagues (too many to name individually) who have helped us in our field collections. ToC
Alfred, E. R., 1961. Singapore Freshwater Fishes . Malay. Nat. J., 15: 1-19.
Alfred, E. R., 1964. Channa bistriata (Weber and de Beaufort), the young of the snake-head fish Channa lucius (Cuvier) . Bull. Natn. Mus. Singapore, 32: 155-156.
Alfred, E. R., 1966. The Fresh-Water Fishes of Singapore . Zool. Verh., Leiden, 78: 1-68, 8 plates.
Alfred, E. R., 1968. Rare and Endangered Freshwater Fishes of Malaya and Singapore . In: Technical Session IV - Threatened Species, Conference on Conservation of Nature and Natural Resources in Tropical South-east Asia, Bangkok, Thailand. IUCN Publications, new series, Number 10, part 4, pp. 325-331.
Bean, B. A. & A. C. Weed, 1912. Notes on a collection of fishes from Java, made by Owen Bryant and William Palmer in 1909, with description of a new species . Washington D.C. Smithsonian Inst. Nat. Hist. Proc., 42: 587-611.
Berg, L. S., 1940. Classification of Fishes, both recent and fossil . Trav. Inst. Zool. Acad. Sci. URSS, 5: 87-345. [In Russian]
Bloch, M. E. & J. G. Schneider, 1801. Systema lchthyologiae Iconibus cx illustratum . Berlin. pp. i-lx, 1-584 Pls. 1-110.
Boeseman, M., 1949. On Pleistocene remains of Ophicephalus from Java, in the 'Collection Dubois' . Zool. Med. 30(6): 83-94.
Breder, C. M. & D. E. Rosen, 1966. Modes of Reproduction in Fishes . The Natural History Press, N.Y., xvi+941 pp.
Cuvier, G. & A. Valenciennes, 1831. Histoire naturelles des Poissons . Vol. 7, Paris-Strasbourg, xxix+531 pp., Pls. 170-208.
Day, F., 1878-1888. The Fishes of India . London, 2 volumes.
Day, F., 1889. Fishes. In: The Fauna of British India, including Ceylon and Burma . Ed. W. T. Blandford, Taylor & Francis, London, Vol. I: i-xviii, 1-548; Vol. II: i-xiv, 1-509.
DeWitt, H. H., 1960. A contribution to the ichthyology of Nepal . Stanford Ichthyol. Bull., 7(4): 63-88.
Deraniyagala, P. E, P., 1929. The Labyrinthici of Ceylon . Spolia Zeylanica, 15(2): 79-111, Pls. 23-31.
Deraniyagala, P. E. P., 1932. Ichthyological Notes. The systematic position of the genus Channa . Spolia Zeylanica, 17(1): 40-41.
Deraniyagala, P. E. P., 1963. The distribution of the genus Channa Gronov 1763 in Ceylon . Spolia Zeylanica, 30: 71-74.
Duncker, G., 1904. Die Fische der malayischen Halbinsel . Mitt. naturh. Mus. Hamburg, 21: 133-207, Pls. 1, 2.
Ettrich, G., 1982. Das Schlangenkopffisch-Männchen entpuppte sich als Maulbrüter . Aquarien Magazin, 16(11): pp. 650-653.
Ettrich, G., 1986. Fische voller Überraschungen . DATZ, 39(7): 289-283.
Ettrich, G., 1989a. Channa gachua aus Südostasien und Channa orientalis von Sri Lanka - zwei gute Arten . DATZ, 42(3): 465-467.
Ettrich, G., 1989b. Breeding the Green Snakehead - It's a mouthbrooder! . Tropical Fish Hobbyist, 37(10): 34-36.
Fowler, H. W., 1938. A list of the fishes known from Malaya . Fish. Bull. Singapore, 1: i-lvi, 1-268.
Gronovius, L. T., 1763. Zoophylacii Gronovianijasciculus primus . Lugduni Batavorum, 136 pp.
Günther, A., 1861. Catalogue of the Acanthopterygian Fishes in the collection of the British Museum. Volume III , 586 pp.
Hamilton-Buchanan, F., 1822. An account of the fishes found in the River Ganges and its branches . Edinburgh and London. pp. i-vii, 1-405, Atlas Pis. 1-39.
Hay, M. S. & I. J. Hodgkiss, 1981. Hong Kong Freshwater Fishes . Urban Council Publication, Hong Kong, 75 pp.
Herre, A. W. C. T. & G. S. Myers, 1937. A contribution to the ichthyology of the Malay Peninsula. Part If. Freshwater fishes . Bull. Raffles Mus., 13; 53-74, Pis. 5-7.
Hora, S. L., 1921. Notes on the occasional absence of the paired fins in fresh-water fishes, with some observations on the two apodal genera Channa Gronow and Apua Blyth . Rec. Ind. Mus., 22: 27-32.
Hora, S. L. & D. D. Mukerji, 1934. Notes on fishes in the Indian Museum. XXII. On a collection of fish from the Southern Shan States and the Pegu Yomas, Burma. Rec. Ind. Mus., 36: 125-138.
Inger, R. F. & P. K. Chin, 1962. The fresh-water fishes of North Borneo . Fieldiana, Zool., 45: 1-268.
Jayaram, K. C., 1981. The Freshwater Fishes of India, Pakistan, Bangladesh, Burma and Sri Lanka - A Handbook . The Zoological Survey of India, 475 pp., 13 pls.
Karoli, J., 1882. Prodromus Piscium Asiac orientalis a domine Joanne Xanthus annis 1869. 70 collectorum . Termesz. Fuzetek Budapest, 5: 147-187.
Kottelat, M., 1989. Zoogeography of the fishes from Indochinese inland waters with an annotated checklist . Bull. Zool. Mus., Univ. Amsterdam, 12(1): 1-55.
Liem, K. F., 1963. The comparative osteology and phylogeny of the Anabantoidei (Telcosti, Pisces) . Illinois Biol. Monogr., 30: 1-149.
Lim, K. K. P., M. Kottelat & P. K. L. Ng, 1990. Freshwater Fish of Ulu Kinchin, Paliang, Malaysia . Malay. Nat. J., 43(4): 314-320.
Lim, K. K, P. Lim, P. K. L. Ng & M. Kottelat, 1990. On a collection of freshwater fishes from EndauRompin, Johore-Pahang, Peninsular Malaysia . Raffles Bull. Zool., 38(1): 31-54.
Lim, K. K. P. & P. K. L. Ng, 1990. A Guide to the Freshwater Fishes of Singapore . Singapore Science Centre, Singapore, 160 pp.
Mohsin, A. K. M. & A. Ambak, 1983. Freshwater Fishes of Peninsular Malaysia . Penerbit Universiti Pertanian Malaysia, 284 pages.
Munro, I. S. R., 1955. The Marine and Freshwater Fishes of Ceylon . Dept. Ext. Affairs, Canberra, 348 1pp., 56 pls.
Myers, G. S. & L. Shapovalov, 1931. On the identity of Ophicephalus and Channa, two genera of labyrinthfishes . Peking Nat. Hist. Bull. , 6: 33-37.
Ng, P. K. L. & K. K. P. Lim, 1989. Rediscovery of the Dwarf Snakehead, Channa gachua (Hamilton, 1822) (Channidae) in Singapore. Raffles Bull. Zool., 37(1 & 2): 172-174. .
Ng, P. K. L. & K. K. P. Lim, 1990. The Black Snakehead, Channa melasoma (Bleeker, 1851) (Channidae): First Record from Singapore. Raffles Bull. Zool., 38(1): 21-24. .
Ng, P. K. L., H. K. Tan & H. P. Ng, 1987. Anabas, the Climbing Perch. Nature Malaysiana, 12(2): 16-19. .
Peters, W. C. H., 1868. Über die von Herm Dr. F. Jagor in dem ostindischen Archipel gesammelten Fische . Monatsber. Akad. Wiss. Berlin, 1868: 254-281, 460-461.
Roberts, T. R., 1989. The Freshwater Fishes of Western Borneo (Kalimantan Barat, Indonesia) . Mem. Calif. Acad. Sci., 14: 1-210.
Scopoli, G. A., 1777. Introductio ad historiam naturalem etc. Pragae, x+506 pp.
Smith, H. M., 1945. The Fresh-Water Fishes of Siam, or Thailand . U. S. Natn. Mus. Bull., 188: 1-622.
Soong, M. K., 1949. Fishes of the Malayan padi-fields. II. Aruan: Serpent-head fishes . Malayan Nat. J., 4(1): 29-3 1, Pl. IV.
Sterba, G., 1973. Freshwater Fishes of the World . Vol 1: 1-456; Vol. 2: 457-877. TFH Pubins.
Tweedie, M. W. F_ 1936. A list of fishes in the collection of the Raffles Museum . Bull. Raffles Mus., 12: 16-28.
Tweedie, M. W. F., 1940. Additions to the collection of fishes in the Raffles Museum . Bull. Raffles Mus., 16: 68-82.
Tweedie, M. W. F., 1950. Notes on Malayan Fresh Water fishes. 2. The species of Channa Scopoli & Ophicephalus) in the collection of the Raffles Museum . Bull. Raffles Mus., 21: 99-105.
Tweedie, M. W. F, 1952a. Malay names of fresh-water fishes . J. Malay. Br. Roy. As. Soc., 25(1): 62-67.
Tweedie, M. W. F., 1952b. Notes on Malayan fresh-water fishes. 5. Malay names . Bull. Raffles Mus., 24: 80-95.
Weber, M. & L. F. de Beaufort, 1922. The Fishes of the Indo-Australian Archipelago . 4: i-xii, 1-410.
Wheeler, A., 1985. The World Encyclopaedia of Fishes . MacDonald Book, London & Sydney, 368 pp, 501 figures. [first edition, 1975]
1 Today C. harcoutbutleri is a valid species. See: Ng, Ng, Britz: Channa harcourtbulteri (Annandael, 1919): a valis species of snakehead (Perciformes: CHannidae) from Myanmar . Back
2 The authors have revalidated C. baramensis themselves: Ng, H.H.; Tan, S.H.; Ng P.K.L. - Revalidation of Channa baramensis (Steindachner 1901), a species of snakehead from northern Borneo. The sarawak museum journal. vol. XLVII, no. 69 (new series), dec. 1995. pp.219-226. Back
3 The authors have revalidated C. cyanospilos themselves: Back
This text was originally published under the above title in: Essays in Zoology, papers commemorating the 40th of the Department of Zoology, National University of Singapore, Singapore by Chou, L.M.; P.K.L. Ng (eds.) 1990; pp. 127-152. ISBN 9971-62-253-X. The authors have granted snakeheads.org the right to publish it on the org's site. The copyright of text and drawings is still with the authors in full amount.
| © 2001 - 2008 snakeheads.org | HOME of this page |